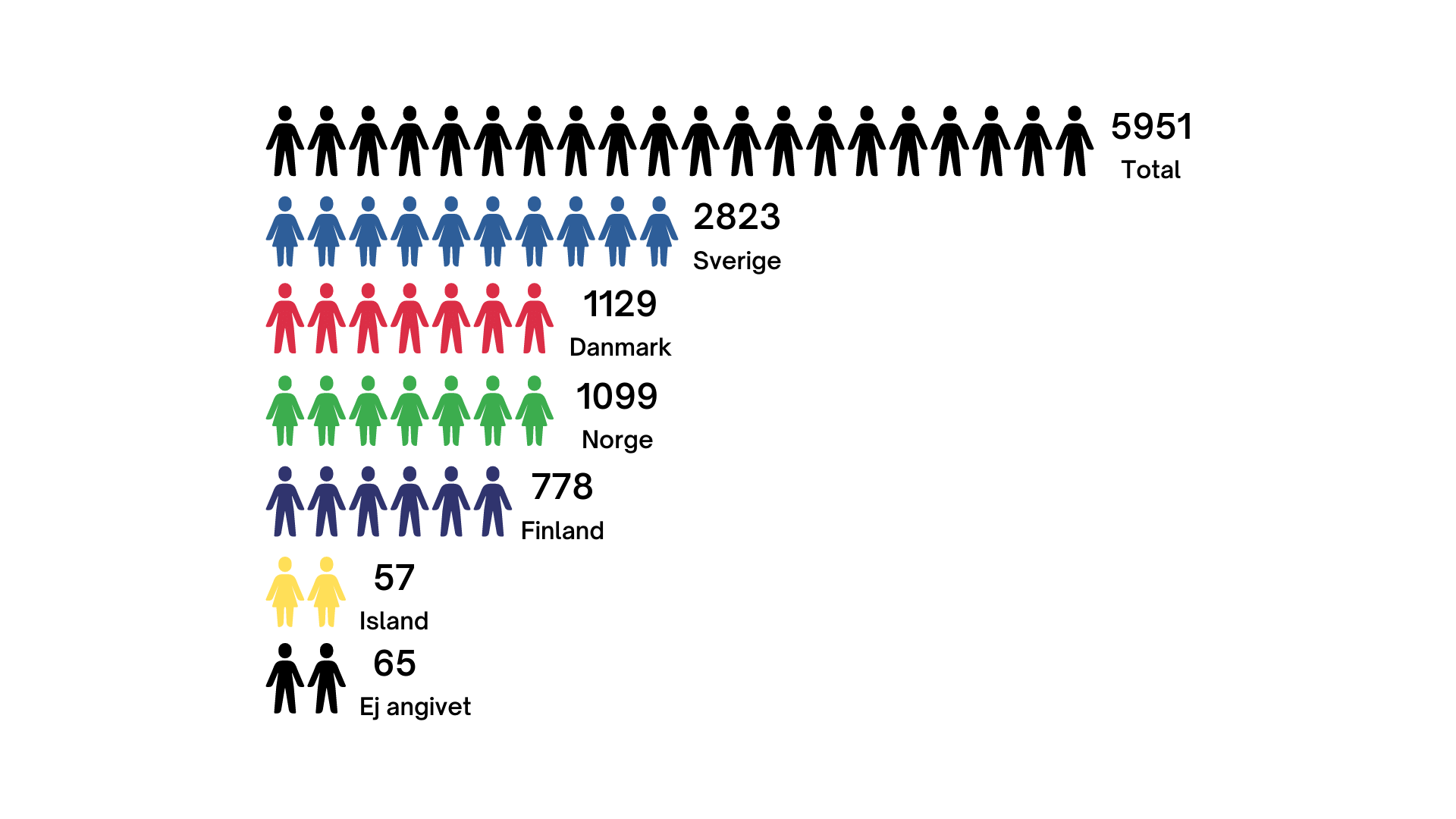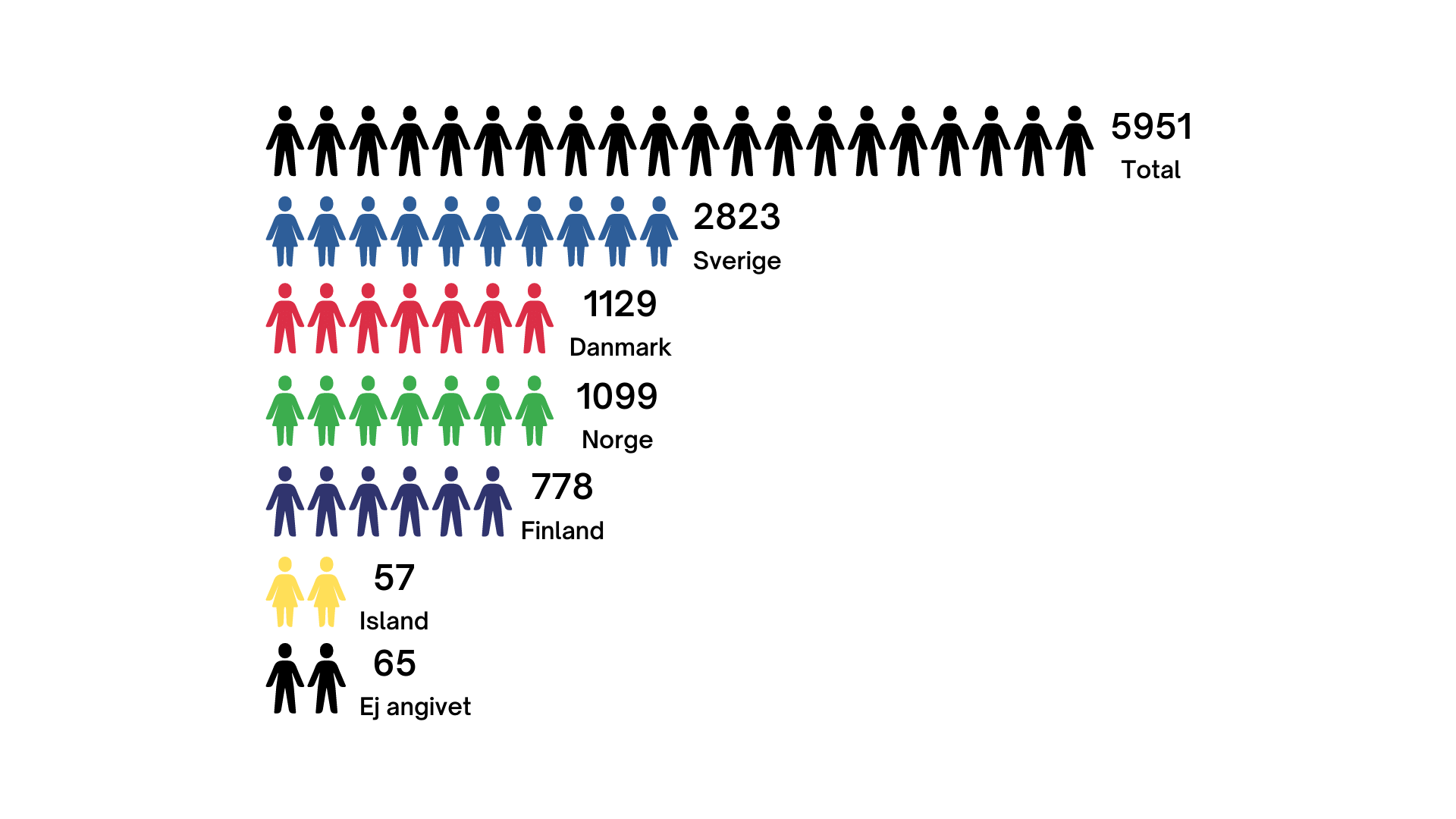Submitting a sample to the biobank
Samples for the NOPHO Leukemia Biobank are taken primarily for use in childhood leukaemia research in the Nordic countries. If necessary, your sample may be used in additional investigations as part of your treatment. Permitted uses for samples stored in a biobank are care and treatment, for example if your healthcare provider needs to re-examine your sample because a treatment is not working as intended, or to make a more reliable diagnosis by comparing new samples with stored ones.
Can biobank material be issued to an insurance company?
No, samples may only be issued for care and approved research in childhood leukaemia in the Nordic countries.
How long is a sample stored if I have given my consent?
Samples in the NOPHO Leukaemia Biobank are stored indefinitely. Consent to store a sample does not guarantee that the sample will be stored. Samples may be discarded if the quality is too poor to be used.
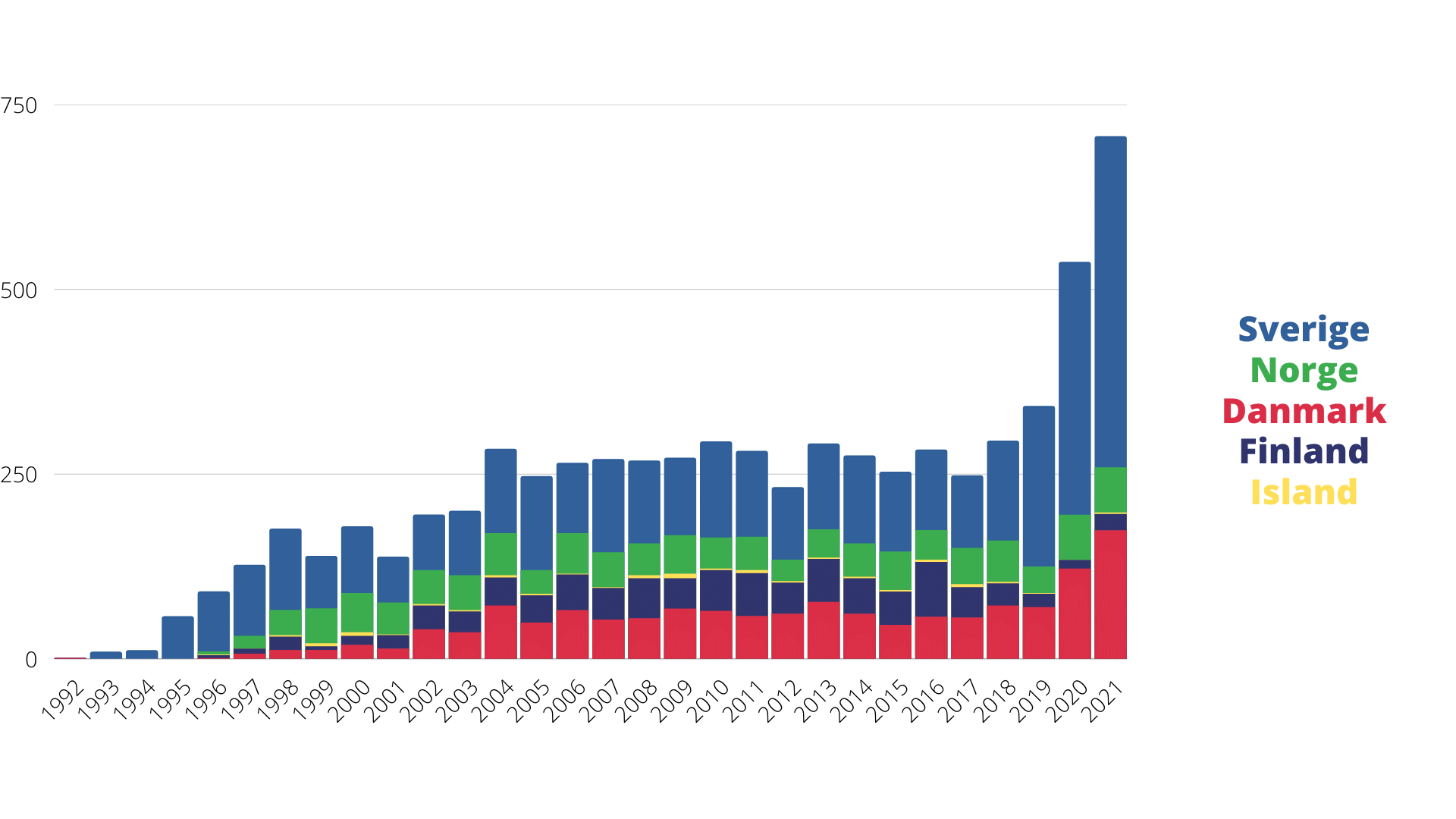

Total number of samples per year and country
The number of samples taken each year since 1992, when childhood leukaemia biobanking was started. In the Nordic region, around 300 children a year contract various types of leukaemia. The increase in sampling in 2020 and 2021 relates to follow-up samples.