Methods for preparing research samples
Cell separation with Ficoll-Paque®
Bone marrow aspirate and peripheral blood are prepared to extract mononuclear leukocytes. Samples are gradient centrifuged with Ficoll-Paque®, a solution that separates cells according to their density or volume mass.
High-density red blood cells fall to the bottom of the centrifuge tube, while mononuclear leukocytes, the white blood cells, end up as a white cloud in the middle.
After being washed in a phosphate buffer, the leukocytes are vitally frozen in a freezing medium that prevents the cells from being damaged.
The proportion of malignant, diseased cells is measured using flow cytometry and entered into the lab data system.
Cells are stored at an extremely low temperature – -198°C – in a cryogenic freezer with liquid nitrogen. Cells can be kept vital – alive – for many years without being significantly affected.
Serum
Serum is centrifuged and aliquoted, divided into 1–4 aliquots and stored at -80°C.
Cerebrospinal fluid
Cerebrospinal fluid is centrifuged and aliquoted, divided into 1–4 aliquots and stored at -80°C.
Extraction of DNA and RNA
Uppsala Biobank uses an extraction robot, QiaCube, and the method is Qiagen Allprep. Link to protocol
DNA and RNA are extracted from cells with high levels of malignant, diseased cells.
DNA and RNA extracts are aliquoted, and are divided into sufficient volumes for various research projects. DNA and RNA are stored at -80°C.
The quality of RNA is measured using a Qubit 4 and entered as a RIN value in the lab data system.
Extraction of constitutional DNA
Whole blood from patients enrolled in the ALLTogether (A2G) study is available for extracting constitutional, congenital DNA.
This is planned to be used for whole-genome sequencing. It can be used to investigate the genetic background of cancer patients and to see whether samples from onset and recurrence differ.
All sample types are divided up into subsamples, or aliquots, so that there is enough for several different analyses.
Cell preparation with Ficoll-Paque®
Cell separation
- Bone marrow aspirate or peripheral blood collected in sodium heparin tubes (might be EDTA).
- Dilute with PBS depending on WBC and at least to double the volume of the sample.
- Centrifuge the Ficoll tubes for 30 minutes at 400g at room temperature.
Collect mononuclear cell fraction, dilute with PBS, centrifuge for 10 minutes at 400g at room temperature.
Vital freezing of mononuclear cells
- Add 4–10 ml PBS and prepare a viable cell count (1:1 toluidine blue).
- Calculate 500,000 cells for flow cytometry = Control % malignant cells. The result of FACS is noted in the Laboratory Information Monitoring System.
- Centrifuge the rest of the cells for 10 minutes at 400g at room temperature.
- The yield of mononuclear cells is distributed into 10 to 30 million cells per cryotube in freezing media (newborn calf serum + 10% dimethyl sulfoxide).
- Place tubes in CoolCell in -80°C freezer for at least 4 hours and at most 7 days.
- Transfer to liquid nitrogen (-198°C) once a week.
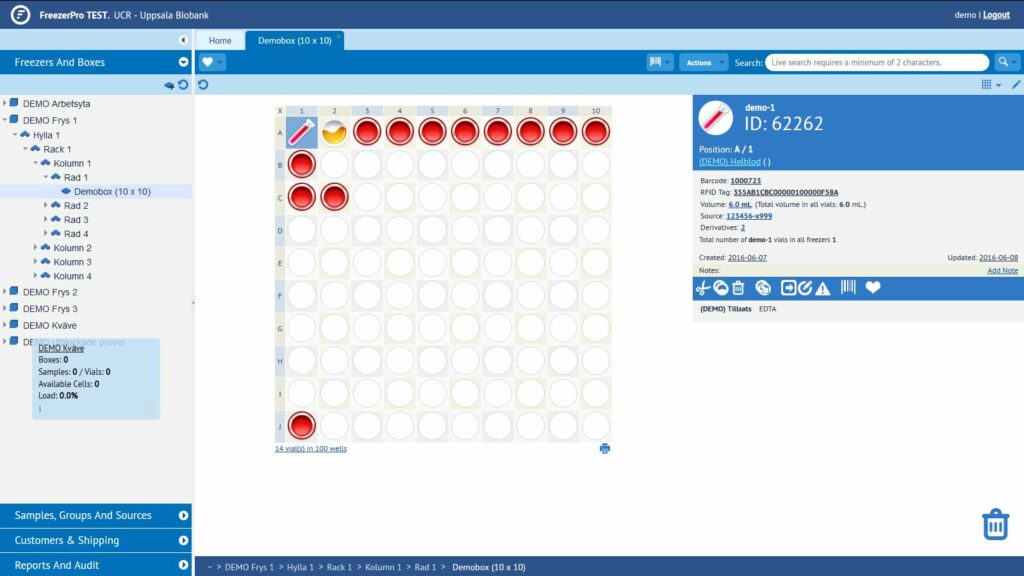
FreezerPro, our lab data system
In the NOPHO Leukemia Biobank, all the information
about the samples is held in a system called FreezerPro. This is a web-based
sample management system that helps to keep track of sample collections and
sample-related data.
The system is a LIMS (Laboratory Information Management System), procured by
Uppsala Biobank for use within Uppsala County Council and Uppsala University.
More information can be found at Uppsala
Biobank – FreezerPro (uu.se)
The image below shows what the system looks like.